
Our work was presented at Johns Hopkins Nano-Bio Symposium. http://inbt.jhu.edu/news/symposium/

Our work was presented at Johns Hopkins Nano-Bio Symposium. http://inbt.jhu.edu/news/symposium/
Expert Rev Med Devices. 2017 Apr 27. doi: 10.1080/17434440.2017.1324293. [Epub ahead of print]
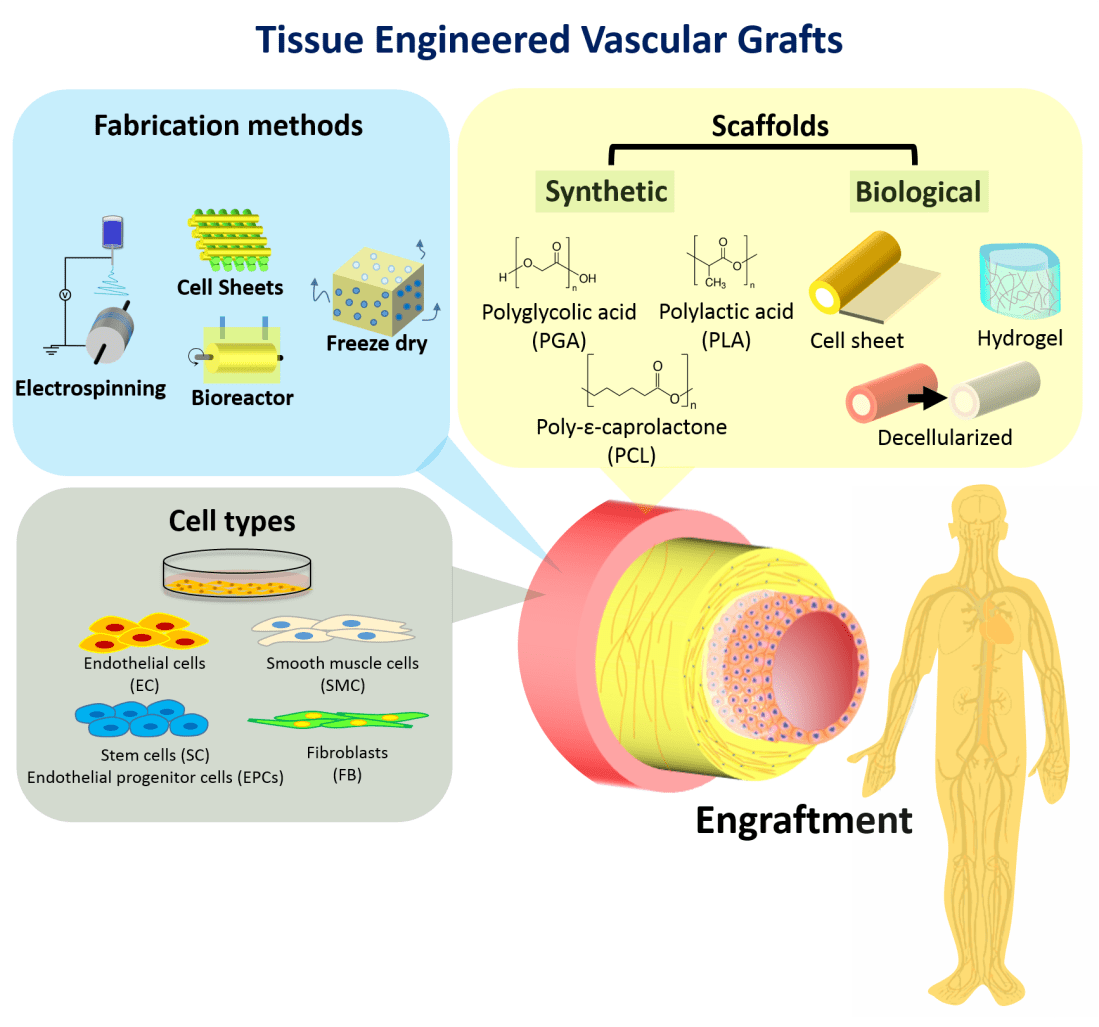
Conventional synthetic vascular grafts are limited by the inability to remodel, as well as issues of patency at smaller diameters. Tissue-engineered vascular grafts (TEVGs), constructed from biologically active cells and biodegradable scaffolds have the potential to overcome these limitations, and provide growth capacity and self-repair.
Areas covered: This article outlines the TEVG design, biodegradable scaffolds, TEVG fabrication methods, cell seeding, drug delivery, strategies to reduce wait times, clinical trials, as well as a 5-year view with expert commentary.
Expert Commentary: TEVG technology has progressed significantly with advances in scaffold material and design, graft design, cell seeding and drug delivery. Strategies have been put in place to reduce wait times and improve “off-the-shelf” capability of TEVGs. More recently, clinical trials have been conducted to investigate the clinical applications of TEVGs.
Biomaterial; Cell seeding; Tissue Engineered Vascular Grafts; Vascular Grafts; Vascular Surgery
DOI: 10.1080/17434440.2017.1324293
New publication coming soon: https://www.jove.com/video/55438/creation-cardiac-tissue-exhibiting-mechanical-integration-spheroids

Title: Role of Bone Marrow Mononuclear Cell Seeding for Nanofiber Vascular Grafts
Authors: Takuma Fukunishi, MD1 # , Cameron A. Best, BA2 # , Chin Siang Ong, MD1 , Tyler Groehl, PhD3 , James Reinhardt, PhD2 , Tai Yi, MD2 , Hideki Miyachi, MD, PhD2 , Huaitao Zhang, BS1 , Toshiharu Shinoka, MD, PhD2 , Christopher K. Breuer, MD2 , Jed Johnson, PhD3 , Narutoshi Hibino, MD, PhD1, *
Affiliations:
1 Department of Cardiac Surgery, John Hopkins University, Baltimore, MD
2 Tissue Engineering and Surgical Research, Nationwide Children’s Hospital, Columbus, OH
3 Nanofiber Solutions, Inc. Columbus, OH
# Equally contributing first authors
Journal: Tissue Engineering Part A
Tissue-engineered vascular grafts (TEVGs) offer potential to overcome limitations of current approaches for reconstruction in congenital heart disease by providing biodegradable scaffolds on which autologous cells proliferate and provide physiologic functionality. However, current TEVGs do not address the diverse anatomic requirements of individual patients. This study explores the feasibility of creating patient-specific TEVGs by combining 3-dimensional (3D) printing and electrospinning technology.
An electrospinning mandrel was 3D-printed after computer-aided design based on preoperative imaging of the ovine thoracic inferior vena cava (IVC). TEVG scaffolds were then electrospun around the 3D-printed mandrel. Six patient-specific TEVGs were implanted as cell-free IVC interposition conduits in a sheep model and explanted after 6 months for histologic, biochemical, and biomechanical evaluation.
All sheep survived without complications, and all grafts were patent without aneurysm formation or ectopic calcification. Serial angiography revealed significant decreases in TEVG pressure gradients between 3 and 6 months as the grafts remodeled. At explant, the nanofiber scaffold was nearly completely resorbed and the TEVG showed similar mechanical properties to that of native IVC. Histological analysis demonstrated an organized smooth muscle cell layer, extracellular matrix deposition, and endothelialization. No significant difference in elastin and collagen content between the TEVG and native IVC was identified. There was a significant positive correlation between wall thickness and CD68+macrophage infiltration into the TEVG.
Creation of patient-specific nanofiber TEVGs by combining electrospinning and 3D printing is a feasible technology as future clinical option. Further preclinical studies involving more complex anatomical shapes are warranted.
Copyright © 2016. Published by Elsevier Inc.
3D printing; Fontan circulation; cell-free tissue engineering; congenital heart disease; electrospun nanofibers; patient-specific; preclinical study; sheep model; tissue-engineered vascular graft