Our biomaterial-free 3D bioprinted cardiac patches got featured at http://medicalphysicsweb.org/cws/article/research/69520.
A shoutout to the author of the article, Geoffrey Potjewyd, for writing about our work. We appreciate it!
Our biomaterial-free 3D bioprinted cardiac patches got featured at http://medicalphysicsweb.org/cws/article/research/69520.
A shoutout to the author of the article, Geoffrey Potjewyd, for writing about our work. We appreciate it!
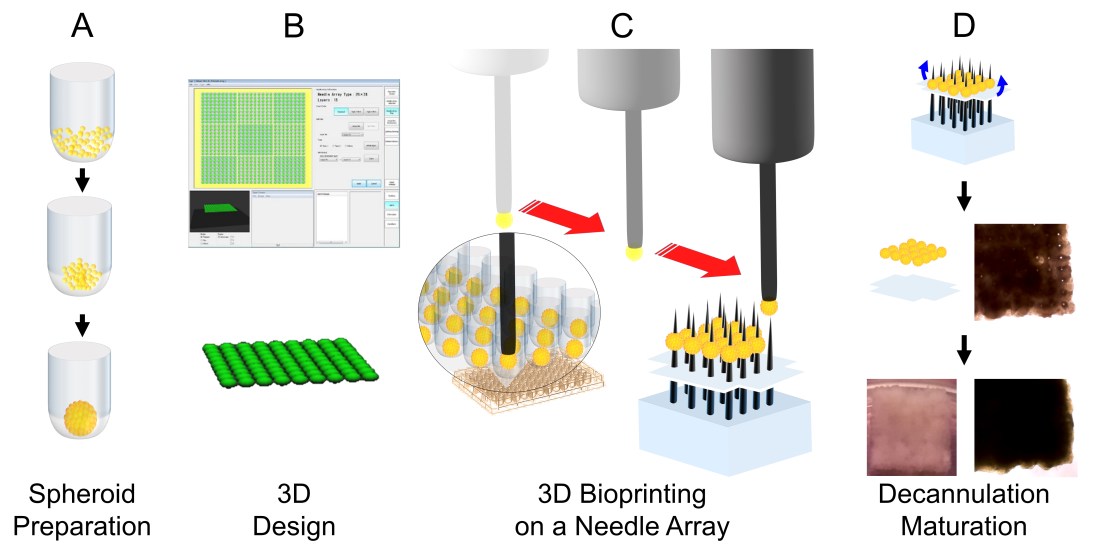
We have developed a novel method to deliver stem cells using 3D bioprinted cardiac patches, free of biomaterials. Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), fibroblasts (FB) and endothelial cells (EC) were aggregated to create mixed cell spheroids. Cardiac patches were created from spheroids (CM:FB:EC = 70:15:15, 70:0:30, 45:40:15) using a 3D bioprinter. Cardiac patches were analyzed with light and video microscopy, immunohistochemistry, immunofluorescence, cell viability assays and optical electrical mapping. Cardiac tissue patches of all cell ratios beat spontaneously after 3D bioprinting. Patches exhibited ventricular-like action potential waveforms and uniform electrical conduction throughout the patch. Conduction velocities were higher and action potential durations were significantly longer in patches containing a lower percentage of FBs. Immunohistochemistry revealed staining for CM, FB and EC markers, with rudimentary CD31+ blood vessel formation. Immunofluorescence revealed the presence of Cx43, the main cardiac gap junction protein, localized to cell-cell borders. In vivo implantation suggests vascularization of 3D bioprinted cardiac patches with engraftment into native rat myocardium. This constitutes a significant step towards a new generation of stem cell-based treatment for heart failure.
Congratulations to Dr Narutoshi Hibino for being awarded the Discovery Award!
Novel high-throughput screening system for cardiac tissue – Narutoshi Hibino (Medicine) & Yun Chen (Engineering)
There remains a need for large animal models to evaluate tissue-engineered vascular grafts (TEVGs) under arterial pressure to provide preclinical data for future potential human clinical trials. We present a comprehensive method for the interrogation of TEVGs, using an ovine bilateral arteriovenous (AV) shunt implantation model. Our results demonstrate that this method can be performed safely without complications, specifically acute heart failure, steal syndrome, and hypoxic brain injury, and it is a viable experimental paradigm. Our method allows for a non-invasive evaluation of TEVGs in terms of graft flow, graft diameter, and graft patency, while also allowing for graft needle puncture under ultrasound guidance. In addition, traditional pathological analysis, histology, and immunohistochemistry may be performed with the contralateral side providing paired control data to eliminate inter-subject variability while reducing the total number of animals. Further, we present a review of existing literature of preclinical evaluation of TEVGs in large animal models as AV conduits.
arteriovenous shunts; electrospinning; large animal models; nanofibers; tissue-engineered vascular grafts; vascular surgery
Congratulations to Dr Narutoshi Hibino for winning The Alfred Blalock Research Award, Johns Hopkins University!